The next generation of materials is providing much-needed protection against chemical and biological warfare agents.
The team at the U.S. Army DEVCOM Soldier Center is focused on developing the next generation of chemical biological (CB) protective materials. We work with collaborators in industry, academia, non-profit research organizations, and other government agencies to improve protection and create multi-functional materials that will enhance current capabilities. Our program, Multi-functional Materials for CB Protective Garments, funded through the Defense Threat Reduction Agency, is developing new CB protective textiles for suits. This work aims to replace or augment carbon as the sorptive medium of CB protective suits, and introduce new capabilities that allow suits to react with toxic chemicals and self-decontaminate.
Using nonwoven materials, we were able to coat fiber surfaces with reactive sorbents, such as zirconium hydroxide and metal organic frameworks (MOFs), to create fabrics with the ability to break down multiple classes of chemical warfare agent.
Carbon has excellent sorption and works well to protect against chemical warfare agents, but it doesn’t have the ability to break down or decontaminate toxic chemicals after they are adsorbed. This means deadly chemicals can be trapped within the suit, and remain a hazard when removing the suit and disposing of it. Adding reactive capabilities will benefit those who have to wear and dispose of CB suits – soldiers, first responders, HAZMAT teams – because we will be able to break down those trapped chemicals and remove a lot of risk.
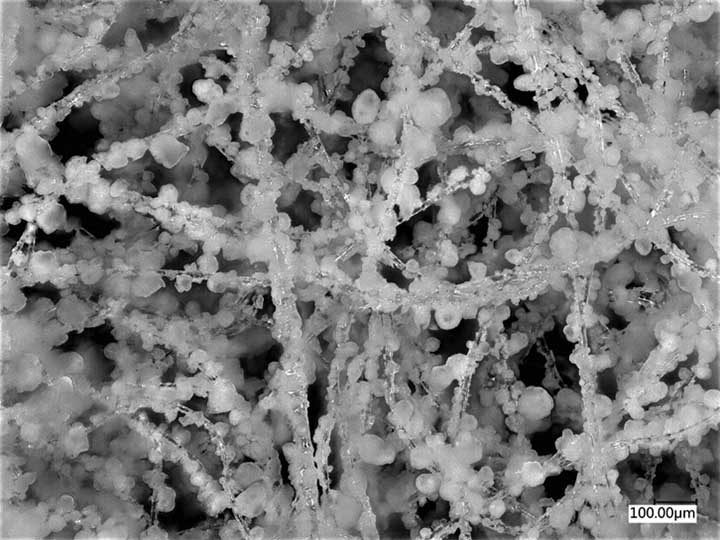
Using nonwoven materials, we were able to coat fiber surfaces with reactive sorbents, such as zirconium hydroxide and metal organic frameworks (MOFs), to create fabrics with the ability to break down multiple classes of chemical warfare agent. There are a lot of challenges in doing this, as the reactive sorbents can be sensitive to heat and pressure, which are often used when processing nonwovens.

Nonwoven materials have a lot of diversity in how they can be processed and what polymers can be used, and we were able to work with the Nonwovens Institute at North Carolina State University to better understand and tailor our processes. In the end, we were able to produce materials using methods that minimized pressure and heat exposure, and created fabrics coated with a variety of zirconium hydroxide and MOF particles.
We tested these for fabrics for sorbent loading and adhesion, performance against chemical warfare agent simulants, and eventually protection against live chemical warfare agents.
We were able to put our new nonwoven materials into a CB textile configuration and demonstrate reactivity with multiple types of chemical warfare agent, where the nonwovens effectively broke down adsorbed agent into less harmful compounds. Those results show a huge promise for integrating into a full CB protective suite. The nonwoven fabrics can be added into a CB garment to augment, or possibly replace, carbon, and will introduce new abilities for CB suits to self-decontaminate toxic chemicals. Ultimately, the materials developed through this program will offer new capabilities in CB protective suits and better protect wearers who rely on them.


